Find Atomic Percentage . the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. a) converting from atomic percent to weight percent: For each element listed in the compound, multiply the atomic percent of. Discover atomic to weight percent. to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. i assume by 'atomic percentages' you mean molar percentages aka molar fractions. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. If $p_i$ is the mass percentage of each. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the.
from chem.libretexts.org
For each element listed in the compound, multiply the atomic percent of. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. Discover atomic to weight percent. a) converting from atomic percent to weight percent: to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. If $p_i$ is the mass percentage of each. i assume by 'atomic percentages' you mean molar percentages aka molar fractions.
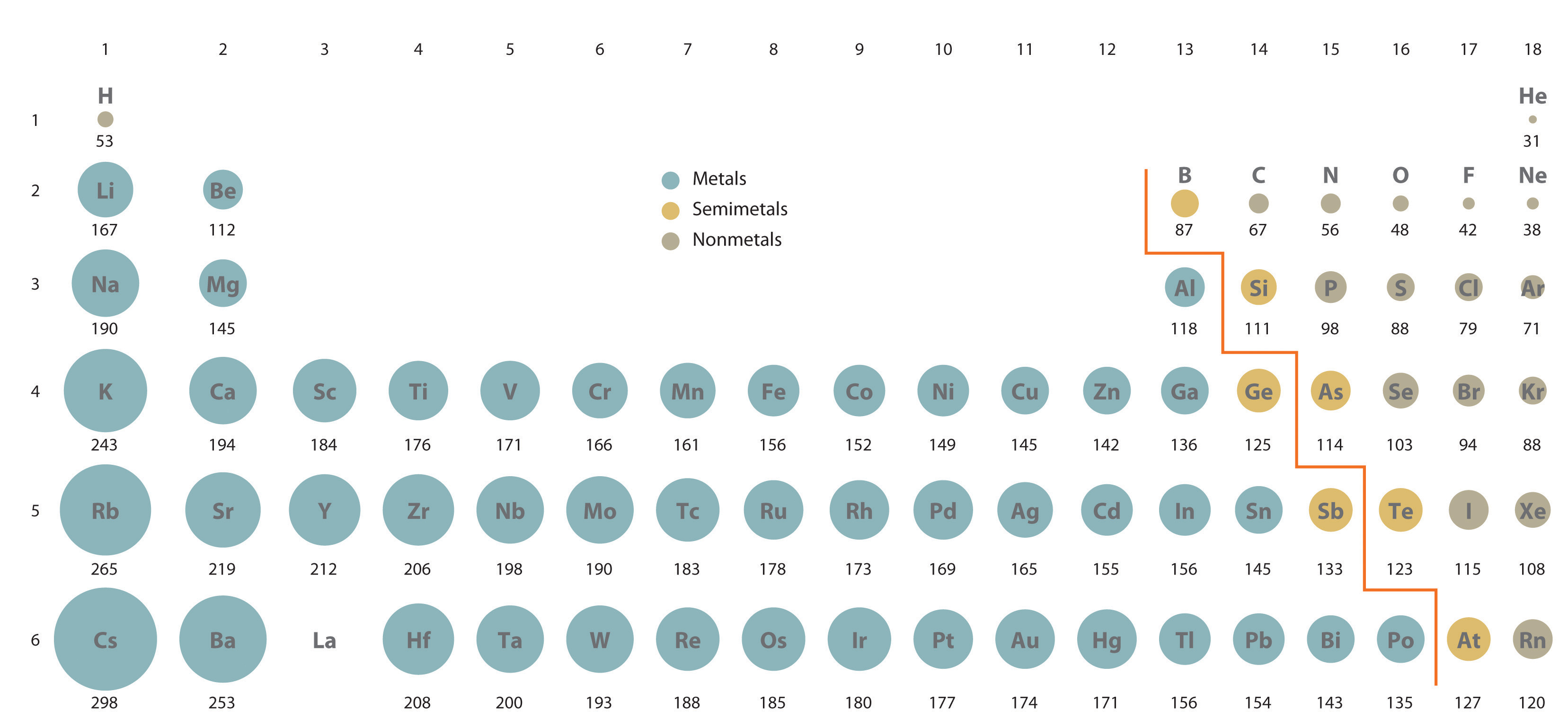
7.3 Sizes of Atoms and Ions Chemistry LibreTexts
Find Atomic Percentage i assume by 'atomic percentages' you mean molar percentages aka molar fractions. the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. a) converting from atomic percent to weight percent: If $p_i$ is the mass percentage of each. Discover atomic to weight percent. i assume by 'atomic percentages' you mean molar percentages aka molar fractions. For each element listed in the compound, multiply the atomic percent of. to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Find Atomic Percentage to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. If $p_i$ is the mass percentage of each. a) converting from atomic percent to weight percent: the atomic mass of. Find Atomic Percentage.
From getrevising.co.uk
Combined Science C1 Atomic Structure. Revision Cards in GCSE Chemistry Find Atomic Percentage i assume by 'atomic percentages' you mean molar percentages aka molar fractions. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. the atomic mass of an. Find Atomic Percentage.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Find Atomic Percentage If $p_i$ is the mass percentage of each. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. i assume by 'atomic percentages' you mean molar percentages aka. Find Atomic Percentage.
From www.printablee.com
Periodic Table With Mass And Atomic Number 10 Free PDF Printables Find Atomic Percentage atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. a) converting from atomic percent to weight percent: For each element listed in the compound, multiply the atomic percent of. so, it is really quite simple to convert from atomic percent to weight percent and. Find Atomic Percentage.
From www.slideserve.com
PPT PERCENT COMPOSITION PowerPoint Presentation, free download ID Find Atomic Percentage For each element listed in the compound, multiply the atomic percent of. a) converting from atomic percent to weight percent: If $p_i$ is the mass percentage of each. i assume by 'atomic percentages' you mean molar percentages aka molar fractions. so, it is really quite simple to convert from atomic percent to weight percent and vice versa.. Find Atomic Percentage.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Find Atomic Percentage a) converting from atomic percent to weight percent: i assume by 'atomic percentages' you mean molar percentages aka molar fractions. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. If $p_i$ is the mass percentage of each. so, it is really quite simple. Find Atomic Percentage.
From www.sliderbase.com
The Atom Presentation Chemistry Find Atomic Percentage so, it is really quite simple to convert from atomic percent to weight percent and vice versa. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. a) converting from atomic percent to weight percent: the atomic mass of an element is the weighted. Find Atomic Percentage.
From chem.libretexts.org
7.3 Sizes of Atoms and Ions Chemistry LibreTexts Find Atomic Percentage Discover atomic to weight percent. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. If $p_i$ is the mass percentage of each. to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. the atomic mass of an element is the. Find Atomic Percentage.
From www.transformationtutoring.com
Percent Abundance and Average Atomic Mass Calculations Guide Find Atomic Percentage a) converting from atomic percent to weight percent: If $p_i$ is the mass percentage of each. For each element listed in the compound, multiply the atomic percent of. the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. so, it is really quite simple to. Find Atomic Percentage.
From www.youtube.com
How to interpret the EDX spectrum of a given elements by weight Find Atomic Percentage the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. a) converting from atomic percent to weight percent: i assume by 'atomic. Find Atomic Percentage.
From www.sliderbase.com
The Atom Atomic Number and Mass Number Isotopes Presentation Chemistry Find Atomic Percentage If $p_i$ is the mass percentage of each. i assume by 'atomic percentages' you mean molar percentages aka molar fractions. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. so, it is really quite simple to convert from atomic percent to weight percent and. Find Atomic Percentage.
From elementtwentyseven.blogspot.com
LIST OF ATOMIC RADIUS AND ATOMIC WEIGHTS OF ELEMENTS BASIC INFORMATION Find Atomic Percentage to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. If $p_i$ is the mass percentage of each. atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. a) converting from atomic percent to weight percent:. Find Atomic Percentage.
From www.abhayjere.com
Calculating Average Atomic Mass Worksheet Find Atomic Percentage Discover atomic to weight percent. the atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. For each element listed in the compound, multiply the atomic percent of. atom. Find Atomic Percentage.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Find Atomic Percentage i assume by 'atomic percentages' you mean molar percentages aka molar fractions. For each element listed in the compound, multiply the atomic percent of. If $p_i$ is the mass percentage of each. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. the atomic mass of an element is the. Find Atomic Percentage.
From www.youtube.com
1.2 Mole fraction and mole percent YouTube Find Atomic Percentage atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. Discover atomic to weight percent. to calculate percent composition, we divide the experimentally derived mass of each element. Find Atomic Percentage.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Find Atomic Percentage a) converting from atomic percent to weight percent: to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. For each element listed in the compound, multiply the atomic percent of. If $p_i$ is the mass percentage of each. Discover atomic to weight percent. atom percent (mole percent) of. Find Atomic Percentage.
From www.youtube.com
What is the easiest way to find the atomic number and mass number Find Atomic Percentage a) converting from atomic percent to weight percent: For each element listed in the compound, multiply the atomic percent of. If $p_i$ is the mass percentage of each. so, it is really quite simple to convert from atomic percent to weight percent and vice versa. the atomic mass of an element is the weighted average of the. Find Atomic Percentage.
From www.periodictableprintable.com
How To Find Atomic Number On Periodic Table 2024 Periodic Table Printable Find Atomic Percentage atom percent (mole percent) of first element in the binary alloy is calculated from mass percent and the atomic masses of the. Discover atomic to weight percent. to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the. so, it is really quite simple to convert from atomic percent. Find Atomic Percentage.